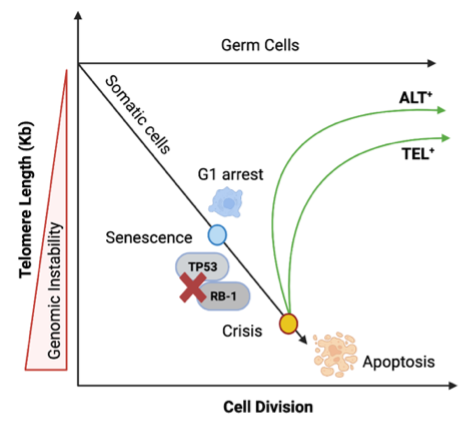
 Telomere length maintenance, genomic instability & cancer
Telomere length maintenance, genomic instability & cancer
Progressive telomere shortening causes heightened DDR machinery recruitment to unprotected chromosome ends driving senescence. Genomic instability allows bypassing senescence checkpoint leading to crisis with critically short telomeres. Cells must either re-express telomerase (TEL+) or use ALT+ pathway to attain replicative immortality and become cancerous.
ALT Cancers:
Of all cancers, 5-10% are ALT+: an increased incidence is seen in mesenchymal tissue-derived paediatric tumours including osteosarcomas, liposarcomas, and neuroepithelial tumours. In fact, one in three solid paediatric tumours are predictably ALT+. Despite overall improvements in targeted cancer therapies, childhood cancers still rely heavily on non-targeted chemotherapies with debilitating side-effects: hampered development, fertility problems and childhood trauma, amongst many others.
Our Vision
Our translationally driven genomic stability and telomere biology research group will strive to identify, understand and exploit therapeutic vulnerabilities in biomarker-defined (ALT+) subsets of childhood cancers to improve both the prognosis and quality of life. We aim to to bridge the gap between discovery and application via a series of interdisciplinary projects with clinicians, chemists and bioinformaticians to tackle the complex problem of developing ALT+ cancer-targeted therapies
Translating Telomeres to Tumors: Discovering Mechanisms, Markers, and Therapies.
Link to google scholar: https://scholar.google.com/citations?hl=en&user=j1UJmEEAAAAJ
Please refer to lab website: https://sites.google.com/view/mayaraghunandanphd/research-vision